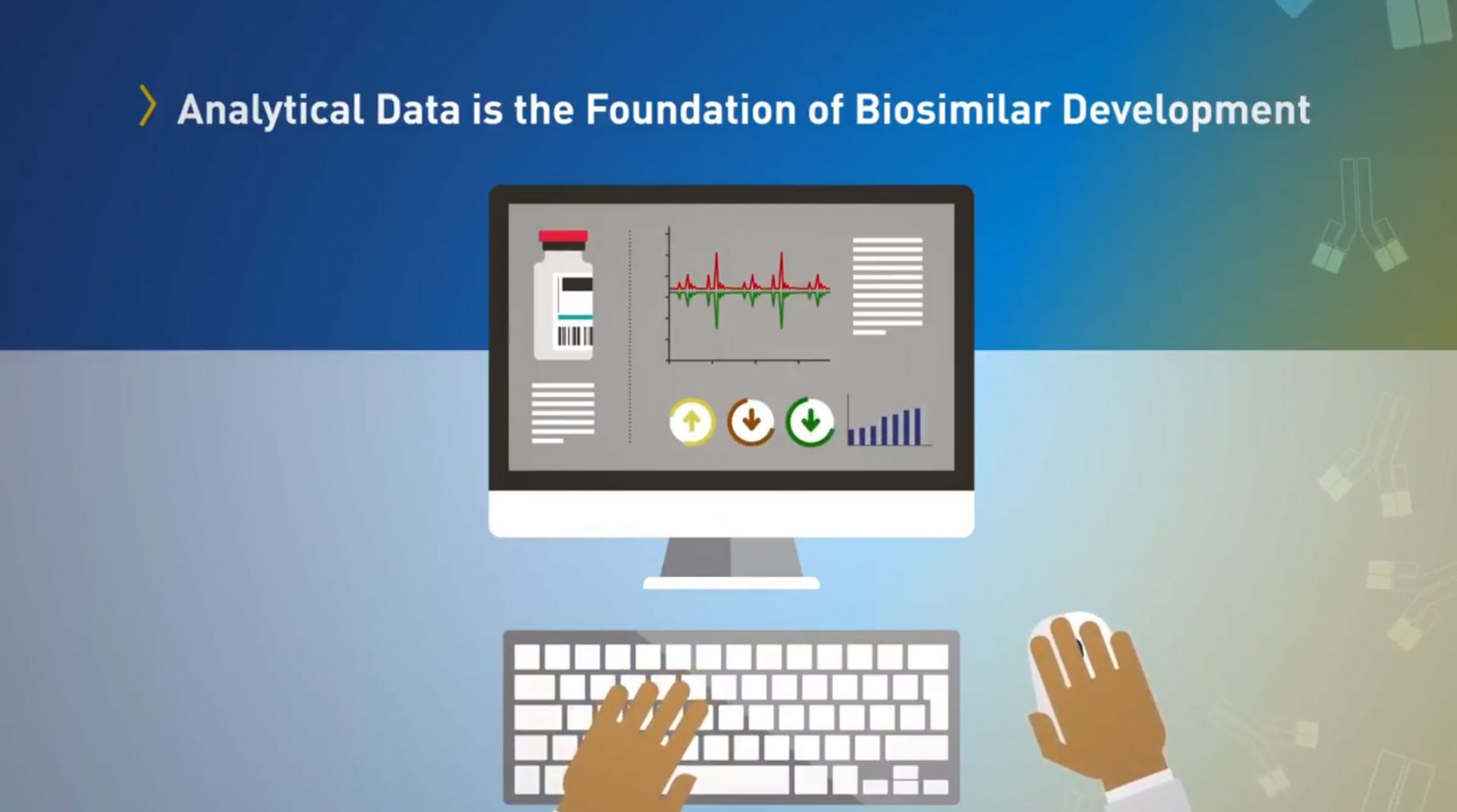
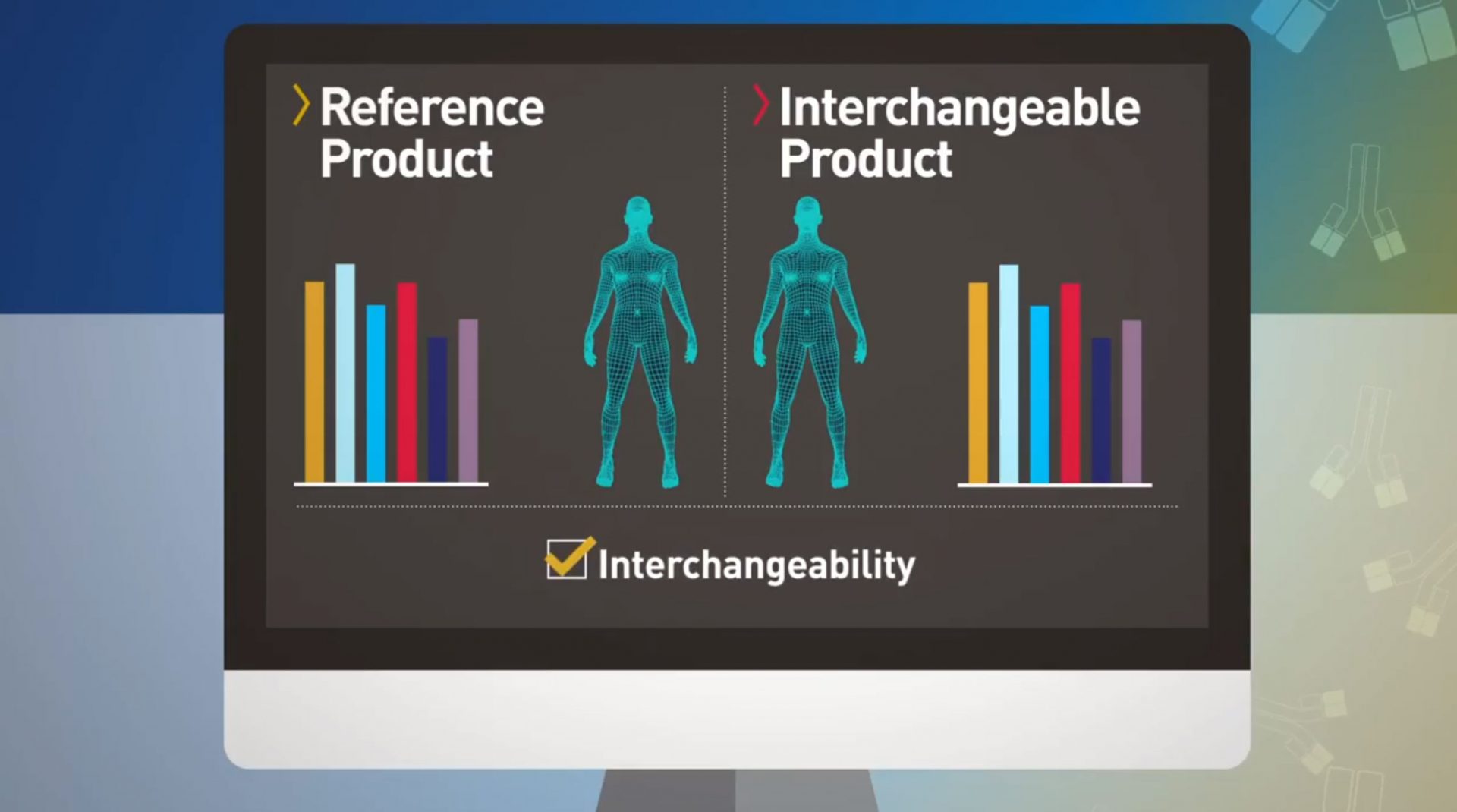
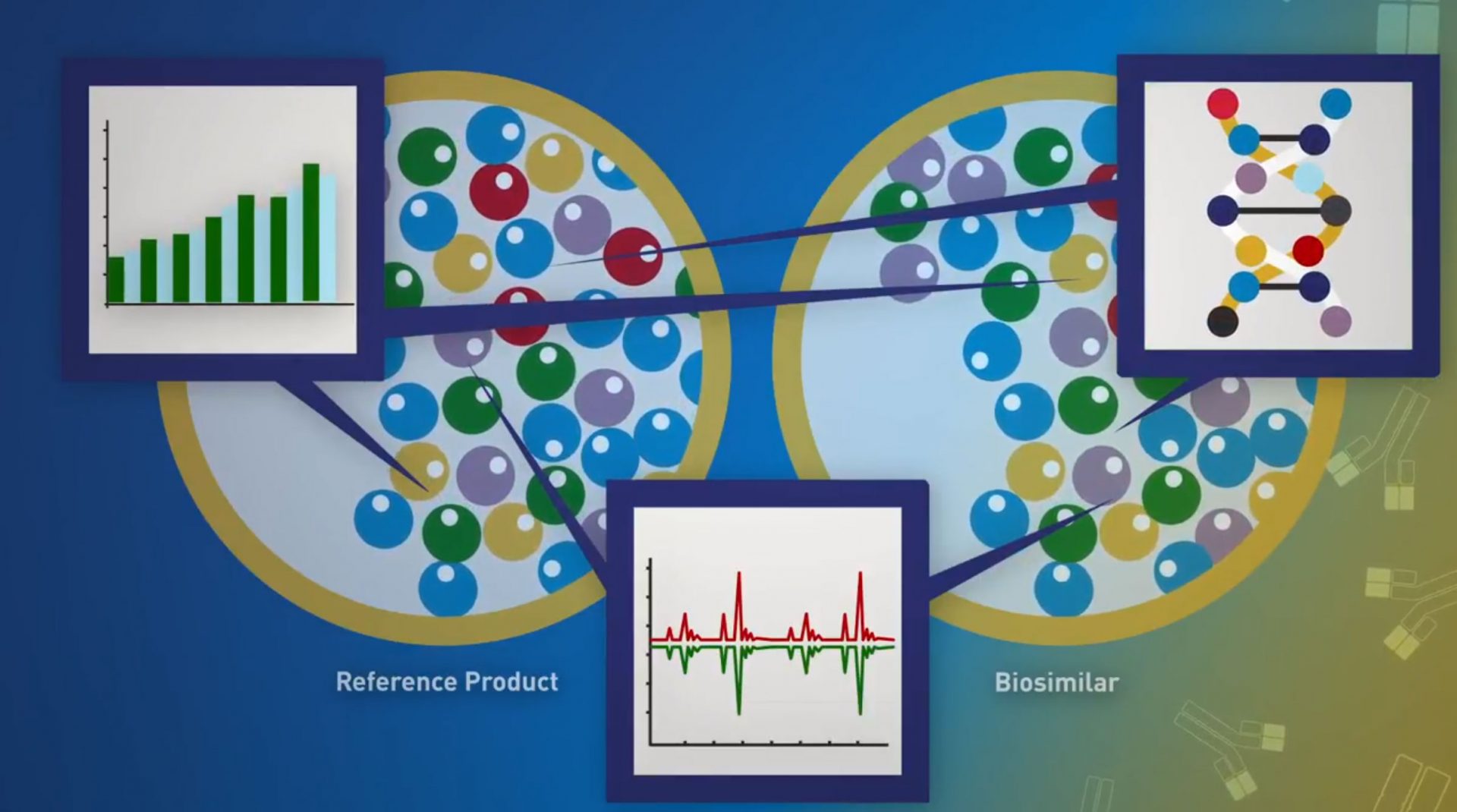
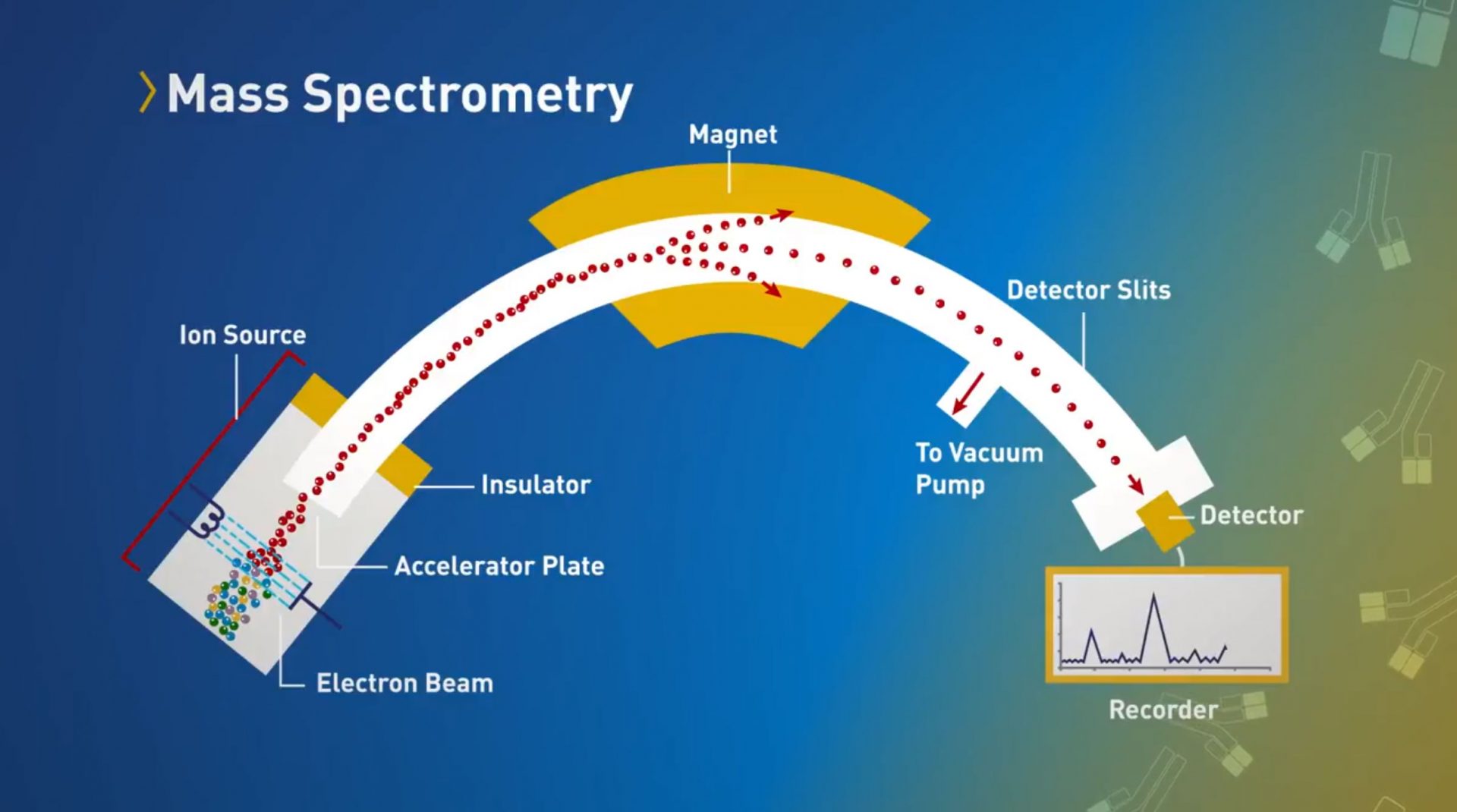
Biological products are the fastest-growing class of therapeutic products in the United States. When patients are prescribed a biological product, biosimilar and interchangeable products can offer additional treatment options, potentially lowering health care costs. FDA tasked out team to develop a campaign to educate stakeholders and the public about biosimilars and the FDA approval process.

Biosimilars
Biosimilar Development, Review and ApprovalClient:
U.S. Food and Drug Administration
Date:
2017
Credits:
Art Director: Simon Fong ▪ Illustrators: Freddy Trejo and Ale Rodriguez-Gitler ▪ Animator/Editor: David Arbor